CRISPR cGMP gRNA Manufacturing
For customers looking to accelerate their CRISPR genome editing program from discovery to clinical trials, our Engineering Run and full cGMP guide RNA manufacturing services offer a streamlined and regulatory-compliant solution. Backed by comprehensive documentation, our guide RNAs simplify regulatory filings, offering a straightforward path to clinical success.
Lab to life changing advances. We’ll help.
Overview
IDT is a global leader in CRISPR genome editing with a complete workflow from design to analysis. Our CRISPR product portfolio includes a comprehensive set of tools, reagents, and services for all your genome editing needs, including discovery, preclinical research, and clinical applications. As your trusted partner, we're committed to providing you with the highest quality products and services to help accelerate your research and bring your discoveries to life.
- Proven excellence: For more than 35 years, IDT has been enabling genomic research with an oligonucleotide manufacturing process unlike anyone else in the industry. Our expertise ensures a reliable partner in your journey from research to clinical applications.
- State-of-the-Art facility: Experience the benefits of our 41,000 square foot Therapeutic Oligonucleotide Manufacturing Facility designed to provide the CRISPR cGMP guide RNA manufacturing grades to align with your development path, supporting you from preclinical studies to clinical trials.
- Comprehensive quality control: We prioritize quality with thorough Quality Control (QC) and analytical testing. Each CRISPR guide RNA meets the highest standards of quality and consistency.
- Regulatory support aligned to clinical phases: IDT is prepared to support you with required information tailored to your unique needs, based on use, clinical program phase, and modality. Navigate regulatory requirements with confidence.
- Dedicated expert team: Your project is in the hands of a dedicated, cross-functional team of professionals at IDT. Each team member is an expert in their field, working diligently to ensure the success of your CRISPR therapeutics program.
Request a consultation
Start the conversation today to explore how our cGMP manufacturing service can accelerate your CRISPR therapeutics project. Click on "request a consultation" to provide brief information about your project, and we'll be in touch to discuss it ASAP.
Product details
IDT’s guide RNAs produced at its Therapeutic Oligonucleotide Manufacturing Facility deliver the high-quality current Good Manufacturing Practices (cGMP) material required for successful completion of your preclinical studies and clinical trials.
Engineering run: Synthetic guide RNA produced by the same manufacturing process as cGMP products but with limited Quality Assurance documentation.
cGMP: Synthetic guide RNA manufactured under cGMP-certified conditions and includes full Quality Assurance Documentation.
| Engineering Run | cGMP | |
|---|---|---|
| Availability | Delivery times after placing the order | Delivery times after placing the order |
| Cleanroom | Certification not required | ISO 8 Clean Room – Certified |
| Changeover | In-place with cleaning validation | In-place with cleaning validation |
| Materials | QA release on raw materials | QA release on raw materials |
| Batch Records | Draft based on customer’s specifications | Based on customer specification |
| Release Testing | Qualified methods | Validated methods |
Through comprehensive quality control and analytical testing, we are committed to ensure that our therapeutic oligonucleotides consistently meet the regulatory requirements to accelerate your project and bring your discoveries to life.
| Category | Attribute | Method |
|---|---|---|
| Identity | Molecular Weight | ESI-MS |
| Purity | Purity | Single-channel CE |
| Process Related Impurities | Elemental Impurities | USP <233> |
| Residual Solvents | USP <467> | |
| Safety | Endotoxin | USP <85> limulus amebocyte lysate (LAL) |
| Bioburden | USP <61/62> | |
| Yield | UV/Vis | Optical Density at 260nm |
| General | Appearance | Visual Inspection |
Product data
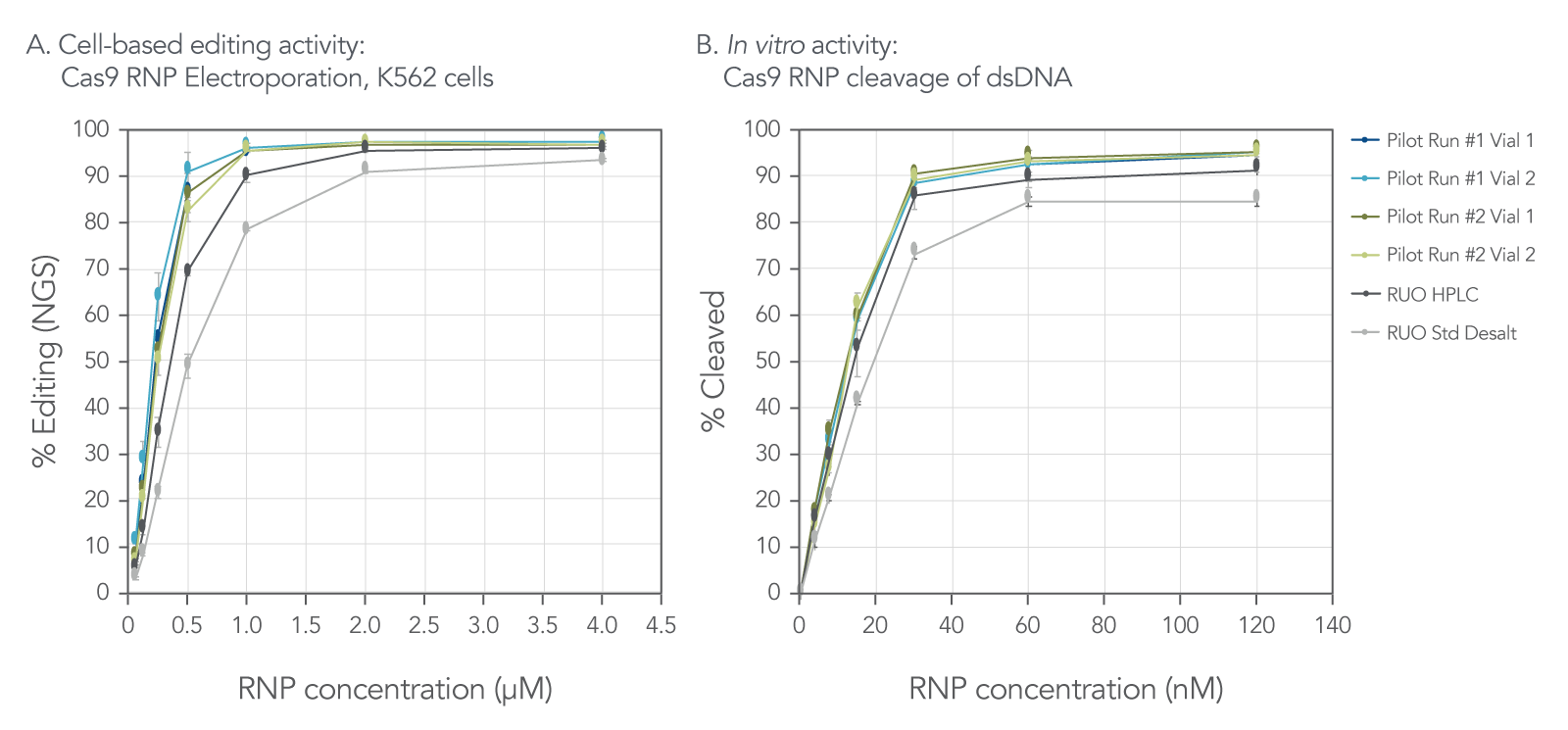
The functional performance of 100 nt Cas9 sgRNAs targeting HPRT1 was studied using a cell-based editing activity assay in K562 cells (a human leukemia cell line) and an in vitro cleavage assay. As shown in Figure 1, pilot-run gRNAs performed better than the guide RNAs with the same sequence but purified using the standard desalt method. However, similar in-cellulo % editing and in vitro % cleavage performance were observed with pilot runs and HPLC-purified guide RNAs. Moreover, different pilot runs exhibited similar functional activity, demonstrating batch-to-batch consistency.
Figure 1. Functional performance of 100 nt Cas9 sgRNAs targeting HPRT1 when used in K562 CRISPR editing and in vitro DNA cleavage experiments. CRISPR editing outcomes were assessed via NGS using the rhAmpSeq™ CRISPR Analysis System following electroporation (Lonza) into K562 cells. Cas9-mediated DNA cleavage in vitro was assessed using the Fragment Analyzer system. (n = 3, error bars represent standard deviation. RUO controls were purified using either HPLC or standard desalt methods).
This data is generated from pilot runs on equivalent Q7 equipment using representative Q7 manufacturing processes, methods, and tests.
Resources
Frequently asked questions
What manufacturing grade of guide RNA products does IDT offer?
What is the difference between IDT’s RUO CRISPR products and those made under cGMP processes?
The ICH Q7 standard IDT used to manufacture cGMP guide RNA can allow these molecules to be used as active pharmaceutical ingredients under specific conditions. The level of change control, validation/verification, record keeping, quality control, and so on is more stringent for this service than for IDT’s research use only (RUO) products.
IDT’s RUO products are manufactured under ISO 9001:2015 conditions to ensure a high-level of quality suitable for research applications.What chemical modifications are available for pre-cGMP (engineering run) and cGMP guide RNAs?
What are the scales and lengths of guide RNAs?
Research use only (RUO), Engineering run and cGMP guide RNAs are available from 20 nt up to 150 nt, at yields ranging from 100 mg up to grams. Custom development options are also available for additional length ranges, modifications, base types, and formulations. Contact us for a consultation to review your project’s needs.
What is the typical QC and analytical testing offered for the Engineering run cGMP and cGMP guide RNA manufacturing services?
IDT offers a comprehensive QC and analytical testing package including identity, purity, yield, endotoxin, bioburden testing, and physical attributes. In addition to standard release testing, IDT will evaluate supporting additional testing on a case-by-case basis. Contact us to discuss your testing requirements.
How do I order Engineering run and/or cGMP guide RNA manufacturing services from IDT?
Does IDT offer regulatory services?
IDT is prepared to support you with required information based on use, clinical program phase and modality. These include but are not limited to drug master files, site master files, site audits, and so on. Contact us for a consultation to discuss your regulatory needs.
cGMP23-2627_001
cGMP gRNA products are manufactured in accordance with ICH Q7. The purchaser is solely responsible for all decisions regarding the intended use of the product and any associated legal or regulatory obligations.


 Processing
Processing